What’s the diagnosis?
A scaly eruption unresponsive to a topical corticosteroid
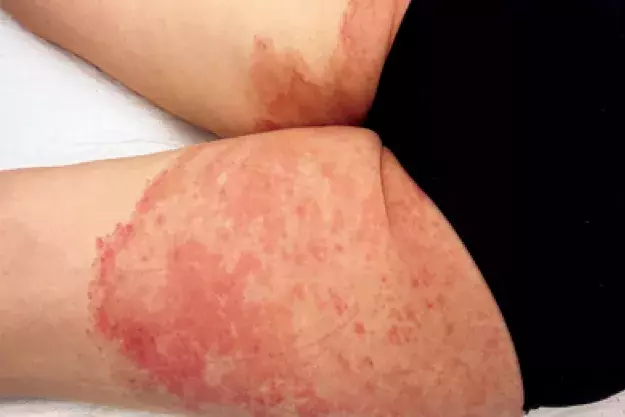
Case presentation
Figures 1a to c.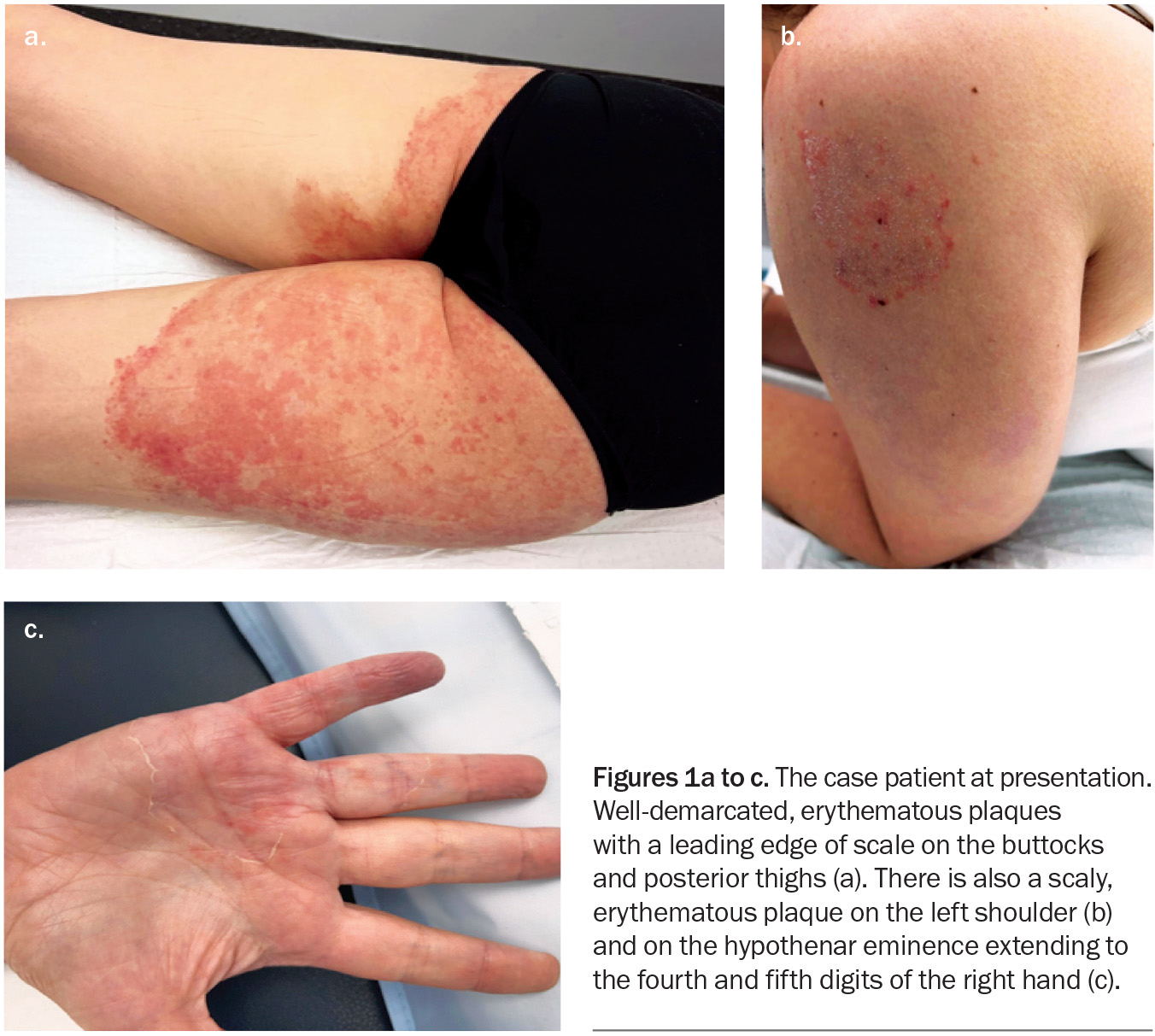
A 41-year-old woman presents with an eight-month history of very itchy, scaly plaques on her buttocks, legs, shoulder and hand (Figures 1a to c). She describes an eruption that began as a single plaque on her posterior thigh with outwardly spreading growth. She has been applying betamethasone dipropionate 0.05% cream, once daily for up to four weeks at a time, to the affected areas. She denies having used soaps or other topical products or medications.
The patient has a history of congenital adrenal hyperplasia, for which she takes prednisolone 8 mg daily, and of anxiety, depression and migraines. She also takes the combined hormonal contraceptive pill (ethinylestradiol 30 mcg/levonorgestrel 150 mcg daily).
On examination, well-demarcated, erythematous plaques with a leading edge of scale are observed on the patient’s buttocks and posterior thighs. Plaques are also observed on her hand and shoulder.
Differential diagnoses
Conditions to consider among the differential diagnoses for a patient presenting with scaly, erythematous plaques include the following.
Seborrhoeic dermatitis
Seborrhoeic dermatitis in adults is a chronic relapsing form of eczema that occurs in areas of active sebaceous glands with high sebum production, such as the face, scalp and trunk.1 It typically presents as well-demarcated plaques that have flaky or branny scale and may be yellow, pink, red or brown in colour.2 Seborrhoeic dermatitis of the face is often symmetrical and can affect the forehead, brows and nasolabial folds.1 It is often mild, with little discomfort or itch. However, the skin can have an increased sensitivity to sun or heat exposure or to topical products, particularly those that contain alcohol.3
Seborrhoeic dermatitis affects an estimated 3% of the population.2,4 The pathogenesis is not fully understood, but it is known that an inflammatory response is associated with overproduction of sebum and growth of the commensal yeast Malassezia.1 The prevalence of adult seborrhoeic dermatitis peaks in the fourth to sixth decades of life and it is more likely to affect men than women.2,4
For the case patient, a diagnosis of seborrhoeic dermatitis was less likely because the lesions were not distributed in areas of high sebum production. In addition, the eruption had been unresponsive to the topical corticosteroid cream and had worsened, whereas seborrhoeic dermatitis would have been expected to improve with topical corticosteroid therapy. Furthermore, severe itch is not a typical feature of seborrhoeic dermatitis.
Allergic contact dermatitis
Allergic contact dermatitis is a delayed (type IV) hypersensitivity reaction localised to the site of contact with an allergen.1 It typically presents as a well-demarcated, pruritic, erythematous eruption with
vesicles, bullae or oedema.5 The margins of the eruption correspond to the edges of the contact area.
Common allergens include nickel, fragrances and methylisothiazolinone (a preservative found in many products, such as wet wipes, cosmetics, hair shampoos and conditioners, body washes, sunscreens and industrial agents) as well as topical medications (neomycin, bacitracin), but there are many allergens in cosmetics and other products that can trigger an eruption.1,5,6 Some gender differences have been attributed to variation in exposure patterns – for example, a predominance for nickel contact dermatitis in women is presumably due to the more frequent wearing of jewellery.1
Allergic contact dermatitis can occur at any age. The pathogenesis is allergen-specific and requires an individual to have had previous exposure to the allergen. The allergen forms an antigen complex when in contact with the skin, which leads to sensitisation. Re-exposure to the allergen elicits a delayed hypersensitivity reaction and cutaneous eruption.5,7 Patch testing is the gold standard method for diagnosis of allergic contact dermatitis.1
For the case patient, allergic contact dermatitis was a possibility to consider because the distribution of lesions could have been explained by allergic contact dermatitis. However, four weeks of treatment with a topical corticosteroid cream would have been expected to clear the eruption, and the lack of response and worsening made this diagnosis less likely.5 The patient was not applying substances that contain common sensitisers. In addition, the eruption did not present with vesicles, bullae or oedema.
Psoriasis
Psoriasis is a chronic inflammatory skin condition with genetic predisposition that can be triggered by environmental factors, such as infection or stress.1,8 The prevalence of psoriasis is approximately 2% in the general population, with the majority of patients being diagnosed before the fifth decade of life.9
The main form of psoriasis is chronic plaque psoriasis, which is characterised by thick, well-demarcated plaques that have an erythematous or salmon-pink colour and silvery scale.1 Lesions can vary in size and distribution. Commonly affected sites include the scalp and the extensor surfaces of the elbows and knees.10 Less common forms may present with varying distributions and plaque characteristics, such as guttate, pustular and flexural (inverse) psoriasis.11
Psoriasis predominantly affects the skin. However, about 20% of patients have associated psoriatic arthritis and there is an association with systemic inflammatory and autoimmune conditions.9,12
The lesions were both erythematous and scaly for the case patient, and psoriasis was considered. However, this diagnosis was less likely because the distribution was not typical of psoriasis. In addition, there was worsening of the condition with the application of topical corticosteroids, whereas psoriasis would have been expected to respond to this treatment and not worsen.
Tinea incognito
This is the correct diagnosis. Tinea incognito is an iatrogenic disease that occurs when a dermatophyte infection is treated inappropriately with topical or systemic corticosteroids.13 The disease has been observed in patients of all ages, with equal gender distribution, and can last for months, or longer, if not detected.14,15 Common causative organisms include Trichophyton rubrum, Trichophyton mentagrophytes and Epidermophyton floccosum.14,16,17
The increasing incidence of tinea incognito is believed to be due to the ease of access to over the counter topical corticosteroids.14 The condition develops during corticosteroid suppression of the normal cutaneous immune response to dermatophytes, which allows the fungal infection to evolve.18,19 Typically, tinea incognito follows a scaly, erythematous, pruritic eruption that has been treated with topical corticosteroids. The irritation and erythema initially improve but when the corticosteroids are ceased, the eruption returns and continues to extend and worsen. By this stage, the eruption no longer presents as a classic dermatophyte infection.15,20 Differences in clinical features of tinea incognito include reduced scale, hypopigmentation, loss of the circular ‘ring’ pattern and hyperactivity at the border.15,18
The emergence of a new, highly inflammatory, terbinafine-resistant species of dermatophyte, Trichophyton indotineae, has been speculated to be the result of inappropriate use of topical corticosteroid and antifungal agents in combination.21 The application of combined corticosteroid and antifungal treatments has been shown to exacerbate dermatophyte growth, which is due to the predominance of the immunosuppressive effects of the topical corticosteroid over the antifungal agent.15
For the case patient, the history of a centrifugal eruption, followed by extension of the eruption after the use of a topical corticosteroid, supported a diagnosis of tinea incognito. A dermatophyte infection was identified from skin scrapings taken from the leading edge of a scaly plaque and fungal culture confirmed the presence of T. rubrum.
Diagnosis
The atypical presentation of tinea incognito means diagnosis is often missed or delayed, and a high index of clinical suspicion is required.16 Mycological examination is very helpful in diagnosis and should be conducted whenever tinea is suspected. The findings may include fungal hyphae and spores, confirming the eruption is caused by a dermatophyte infection.14,16 A thorough examination of the feet and nails should be undertaken, since T. rubrum can also infect the nails and may be the source of the infection.
Management
The management of tinea incognito is similar to that of regular dermatophyte infections, with the additional requirement for cessation of any topical corticosteroids.16 The Australian Therapeutic Guidelines suggest that oral antifungal therapy is appropriate for tinea that has been inappropriately treated with a topical corticosteroid.22 For infection caused by T. rubrum, T. mentagrophytes or E. floccosum, treatment options (adult doses) include:
- terbinafine 250 mg once daily for two weeks
- fluconazole 150 mg once weekly for six weeks
- either itraconazole (Sporanox capsules) 100 mg once daily for two weeks or itraconazole (Lozanoc capsules) 50 mg once daily for two weeks (not bioequivalent)
- griseofulvin 500 mg once daily for eight to 12 weeks.
If there is nail involvement then a longer course of treatment is required, usually 12 weeks but sometimes longer.
For terbinafine-resistant T. indotineae, the treatment of choice has been identified as itraconazole 100 mg twice daily for four to eight weeks, and for up to 12 weeks.23,24 Expertise from infectious disease colleagues should be sought in these cases.
Side effects of systemic oral antifungal therapy include liver dysfunction, nausea, vomiting, constipation, diarrhoea, loss of taste and cutaneous reactions.25,26
Nonpharmacological measures to manage dermatophyte infections include the wearing of loose-fitting clothing made of breathable material (e.g. cotton) that keeps moisture away from the body, as well as ensuring the skin has completely dried after bathing or showering.27
Clinical follow up should show visible improvements in the cutaneous eruption and eradication of the dermatophyte.
Outcome
The case patient was diagnosed with tinea incognito caused by T. rubrum. She was treated with oral terbinafine 250 mg once daily for two weeks, which did not interact with her existing medications. At follow up four weeks later, there was complete clearance of the dermatophyte infection. MT
COMPETING INTERESTS: None.
References
1. Bolognia J, Schaffer JV, Cerroni L. Dermatology. 4th ed. Philadelphia, PA: Elsevier, 2018.
2. Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investig Dermatol 2015; 3.
3. Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician 2015; 91: 185-190.
4. Zander N, Sommer R, Schäfer I, et al. Epidemiology and dermatological comorbidity of seborrhoeic dermatitis: population-based study in 161 269 employees. Br J Dermatol 2019; 181: 743-748.
5. Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician 2010; 82: 249-255.
6. Krob HA, Fleischer AB Jr, D’Agostino R Jr, et al. Prevalence and relevance of contact dermatitis allergens: a meta-analysis of 15 years of published T.R.U.E. test data. J Am Acad Dermatol 2004; 51: 349-353.
7. Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res 2020; 162: 105282.
8. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol 2008; 58: 826-850.
9. Christophers E. Psoriasis − epidemiology and clinical spectrum. Clin Exp Dermatol 2001; 26: 314-320.
10. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009; 361: 496-509.
11. Clarke P. Psoriasis. Aust Fam Physician 2011; 40: 468-473.
12. Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol 2019; 80: 251-265.e19.
13. Ive FA, Marks R. Tinea incognito. Br Med J 1968; 3: 149-152.
14. Kim WJ, Kim TW, Mun JH, et al. Tinea incognito in Korea and its risk factors: nine-year multicenter survey. J Korean Med Sci 2013; 28: 145-151.
15. Dhaher S. Tinea incognito: clinical perspectives of a new imitator. Dermatol Reports 2020; 12: 8323.
16. Nowowiejska J, Baran A, Flisiak I. Tinea incognito – a great physician pitfall. J Fungi (Basel) 2022; 8: 312.
17. Romano C, Maritati E, Gianni C. Tinea incognito in Italy: a 15-year survey. Mycoses 2006; 49: 383-387.
18. Arenas R, Moreno-Coutiño G, Vera L, Welsh O. Tinea incognito. Clin Dermatol 2010; 28: 137-139.
19. Romano C, Asta F, Massai L. Tinea incognito due to Microsporum gypseum in three children. Pediatr Dermatol 2000; 17: 41-44.
20. Marks R. Tinea incognito. Int J Dermatol 1978; 17: 301-302.
21. Moreno-Sabater A, Normand AC, Bidaud AL, et al. Terbinafine resistance in dermatophytes: a French multicenter prospective study. J Fungi (Basel) 2022; 8: 220.
22. Treatment of tinea. In: Therapeutic Guidelines. Melbourne: TG; 2023. Available online at: https://www.tg.org.au (accessed September 2023).
23. Uhrlass S, Verma SB, Graser Y, et al. Trichophyton indotineae – an emerging pathogen causing recalcitrant dermatophytoses in India and worldwide – a multidimensional perspective. J Fungi (Basel) 2022; 8: 757.
24. Rajagopalan M, Inamadar A, Mittal A, et al. Expert Consensus on The Management of Dermatophytosis in India (ECTODERM India). BMC Dermatol 2018; 18: 6.
25. Ellis D, Watson A. Systemic antifungal agents for cutaneous fungal infections. Aust Prescriber 1996; 19: 72-75.
26. Jacobs JA, Kolbach DN, Vermeulen AHM, Smeets MH, Neuman HA. Tinea incognito due to Trichophytom rubrum after local steroid therapy. Clin Infect Dis 2001; 33: e142-e144.
27. Sahoo AK, Mahajan R. Management of tinea corporis, tinea cruris, and tinea pedis: a comprehensive review. Indian Dermatol Online J 2016; 7: 77-86.
Fungal infections

