What’s the diagnosis?
A woman with a pruritic perianal plaque
Case presentation
Figure 1.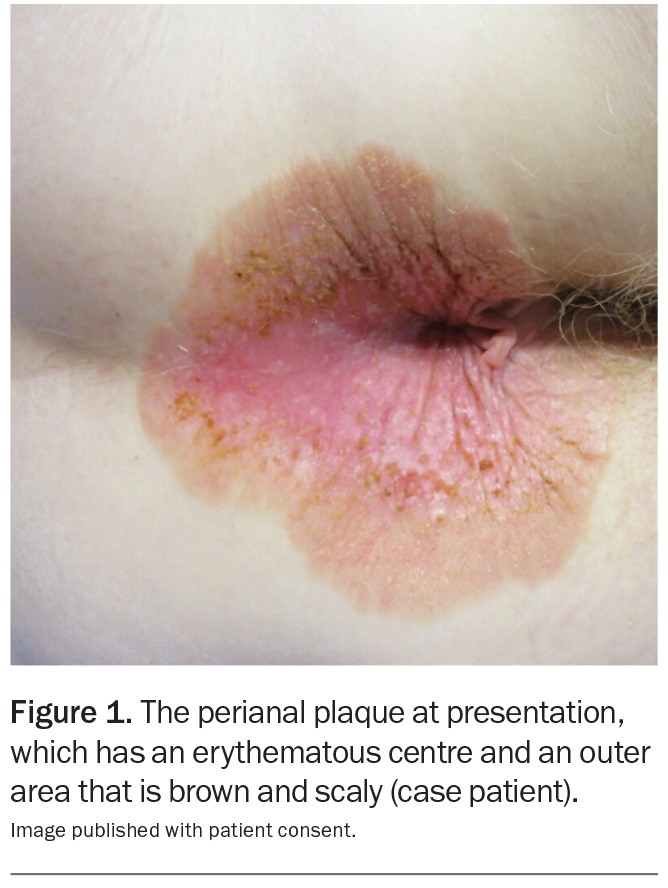
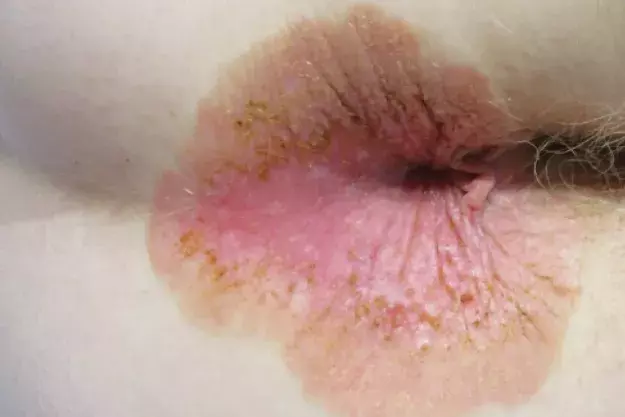
A 69-year-old Caucasian woman presents with a five-month history of a pruritic perianal plaque (Figure 1). The eruption has been treated with an emollient containing zinc oxide as well as topical corticosteroids and topical antifungal therapy but has not responded. The patient has not applied soap, baby wipes or other topical agents (such as a haemorrhoid cream) to the affected area. She does not have any constitutional symptoms, such as diarrhoea or abdominal pain.
The patient has a history of depression, which is managed with citalopram. She has no personal or family history of malignancy or dermatological conditions. She does not have any known allergies.
On examination, a well-demarcated plaque is observed that extends from the anal verge into the perianal area. The plaque is erythematous in its centre and has an outer area that is brown and scaly. There are occasional erosions and a small skin tag. Keratotic brown papules are scattered through the plaque. The patient has no similar lesions elsewhere on her body.
Differential diagnoses
Conditions to consider among the differential diagnoses for a patient who has a perianal plaque include the following.
Psoriasis
Psoriasis is a common, chronic, inflammatory skin condition with an estimated global prevalence of 0.5 to 11% in adults and up to 1.4% in children.1 It has a bimodal distribution for age of disease onset, with incidence peaks at 30 to 39 years and at 50 to 69 years.2,3
The pathogenesis of psoriasis is primarily T-cell mediated, with dendritic cells, TNF-alfa and the interleukin IL-17/ IL-23 axis playing major roles.4,5 Environmental and behavioural factors, such as infection, stress and smoking, can trigger psoriasis in genetically-predisposed individuals.6,7 In a Spanish cross-sectional study of 1022 patients diagnosed with moderate to severe psoriasis, nearly 50% of participants reported a family history of the condition.8
Psoriasis is most commonly recognised as sharply-demarcated erythematous plaques with overlying silvery scale that are distributed symmetrically on extensor surfaces, such as the elbows and knees. However, it can also affect the ‘inverse’ (flexural) areas, such as the axillae and inguinal, genital, perianal and inframammary regions.
This was not the correct diagnosis for the case patient. The erythematous appearance of the plaque could have supported a diagnosis of psoriasis; however, the brown colour differed from the typical salmon-pink of psoriasis and there was a lack of silvery scale. She had no other signs of psoriasis, such as nail abnormalities (onycholysis, nail pits) or other plaques elsewhere on her body. In addition, psoriasis is very responsive to topical corticosteroids, so some improvement would have been expected with this treatment.
Allergic contact dermatitis
Allergic contact dermatitis is a delayed type 4 hypersensitivity reaction mediated by T cells.7 Its pathogenesis requires an individual to have had prior exposure to an allergen: the initial exposure leads to sensitisation; re-exposure elicits a delayed hypersensitivity reaction and cutaneous eruption.
Allergic contact dermatitis typically presents as a pruritic, erythematous scaly eruption with vesicles, bullae and oedema.9 The distribution can provide a valuable clue in identifying the causative allergen – for example, nickel allergy may be suggested by lesions located in the neckline (necklace), submammary region (bra underwire), unilateral periauricular area (mobile phone), wrist (bracelet, watch) or lower abdomen (button on jeans).10 In the perianal region, possible allergens include excipients of soap (fragrances, preservatives) and baby wipes (preservatives such as methylchloroisothiozolinone) as well as haemorrhoid creams (framycetin, cinchocaine).11,12 Patch testing is the gold standard for diagnosis of allergic contact dermatitis.7
This was not the correct diagnosis for the case patient. Although there were aspects of the appearance of the lesion that may have supported this diagnosis, there were irregularities. The rash of allergic contact dermatitis is typically more erythematous (rather than brown), not as well-demarcated and not scaly. Also, the patient’s medical history was not suggestive of the diagnosis, as she had not been applying any substances to the area. Antifungal products may contain sensitising preservatives such as benzyl alcohol, but she had not been applying these consistently (and commenced after development of the problem). Further, topical corticosteroid therapy would have been expected to improve or clear allergic contact dermatitis.9
Tinea cruris
Tinea cruris (‘jock itch’) is a fungal infection affecting the groins, with occasional extension onto the gluteal and abdominal regions. Common causative pathogens include Trichophyton rubrum, Epidermophyton floccosum and Trichophyton mentagrophytes.7 Predisposing factors include the presence of tinea pedis and a history of obesity, diabetes or immunodeficiency. It is more common in males than females and risk is increased when there is excessive sweating.7,13
The early presentation of tinea cruris is usually a thin, pruritic, erythematous plaque in the groin, with a border that is well-demarcated and scaly and may contain pustules and vesicles. Over time, there can be central clearing, hyperpigmentation and extension to the surrounding areas. Skin scrapings for potassium hydroxide staining and fungal culture can be of assistance.14
This is not the correct diagnosis for the case patient. The appearance of her lesion is not typical for tinea cruris (the plaque did not have a leading scaly edge) and it had not responded to topical antifungal therapy. In addition, it would be unusual for a plaque to be present in the perianal region only, without originating in the groins or inner thighs.
Extramammary Paget’s disease
This is the correct diagnosis. Extramammary Paget’s disease (EMPD) is a rare intraepithelial adenocarcinoma that usually affects apocrine-rich regions such as anogenital and axillary skin. In a registry study conducted in the Netherlands, its incidence was estimated to be about 0.11 per 100,000 person-years.15 It most commonly affects individuals over 50 years of age. The prevalence of EMPD in men and women has been shown to differ for Western and Asian populations: there is a predominance in females in Caucasian populations (female:male 3:1), whereas there is a predominance in males in Asian populations.15,16
The pathogenesis of EMPD is not well understood. The disease can be primary (>75% of cases) or secondary and associated with an underlying malignancy.7 In primary EMPD, it has been proposed that cutaneous adnexa, Toker cells, pluripotent stem cells or anogenital mammary-like glands may be sites of origin.17,18 Secondary EMPD is thought to arise from epidermotropic spread of malignant cells from an underlying neoplasm.19
Patients with EMPD may be asymptomatic but usually report pruritus or a burning sensation. A typical lesion is a slowly-expanding, sharply-demarcated erythematous plaque, often with scattered areas of white scales and erosion, resulting in a ‘strawberries and cream’ appearance.7 Secondary changes due to excoriation may also be present, such as crust, lichenification and hyperpigmentation.
The plaque presented in the case displays most of the features described above. A crucial feature in the patient’s history was the lack of response to topical corticosteroids or antifungal therapy, thus warranting further investigations and consideration of EMPD. The patient’s age, sex and ethnicity supported the diagnosis.
Management
The diagnosis of EMPD must be confirmed by biopsy. The distinctive histopathological features are pagetoid cells containing prominent nuclei and vacuolated cytoplasm.20 Immunohistochemical staining should be performed to exclude pagetoid melanoma and intraepithelial neoplasia and to assist in differentiating between primary and secondary EMPD.21 Primary EMPD is usually positive for marker CK7, negative for CK20 and positive for GCDFP-15, whereas secondary EMPD is negative for CK7, positive for CK20 and negative for GCDFP-15.22 Patients with a confirmed diagnosis of EMPD should be evaluated for the possibility of an underlying malignancy.
For a cutaneous EMPD lesion, the primary goal is complete clearance while preserving function and cosmesis. Treatment options include surgical removal (wide local excision, Mohs micrographic surgery), topical imiquimod (off-label use), radiotherapy and carbon dioxide laser therapy.23 EMPD has a low potential for metastasis; however, once metastasised it behaves aggressively and has a poor prognosis (five-year survival rate of 7% for metastatic disease vs 84% in the absence of metastasis).24 Metastatic disease may be treated with chemotherapy, immune checkpoint inhibitors or targeted therapy.23
Outcome
A biopsy was performed for the case patient and confirmed the diagnosis of EMPD. She underwent investigations for underlying malignancy, which included a CT scan of her chest, abdomen and pelvis, a whole-body PET scan, mammography, colonoscopy and blood tests for tumour markers (HE4, CA-125, CA-15.3, CA-19.9 and CEA). The results were all within normal limits.
The patient was referred to a surgeon and a radiation oncologist for advice on management options. Radiation therapy was favoured for preservation of anorectal function, and a course of treatment (66 Gray in 33 fractions over six and a half weeks) was successful (radiation dose derived from Hata, et al).25
Figure 2.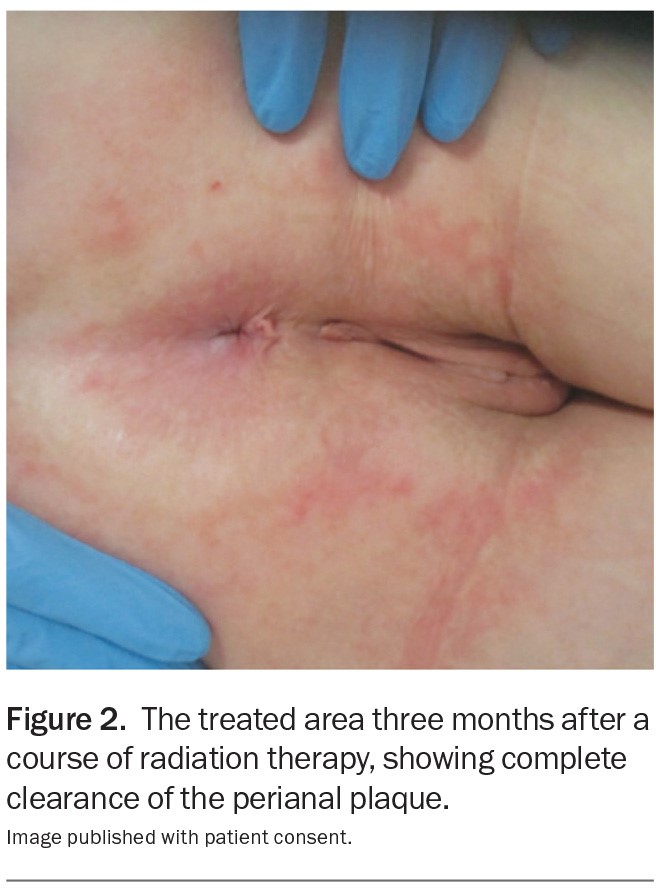
At a follow-up visit three months later, the cutaneous plaque had cleared and the perianal area had healed well (Figure 2). The patient continues to be monitored regularly for disease recurrence. MT
COMPETING INTERESTS: None.
References
1. Michalek IM, Loring B, John S. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol 2017; 31: 205-212.
2. Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013; 133: 377-385.
3. Iskandar IYK, Parisi R, Griffiths CEM, Ashcroft DM; Global Psoriasis Atlas. Systematic review examining changes over time and variation in the incidence and prevalence of psoriasis by age and gender. Br J Dermatol 2021; 184: 243-258.
4. Vo S, Watanabe R, Koguchi‐Yoshioka H, et al. CD8 resident memory T cells with interleukin 17A‐ producing potential are accumulated in disease‐naïve nonlesional sites of psoriasis possibly in correlation with disease duration. Br J Dermatol 2019; 181: 410-412.
5. Phadungsaksawasdi P, Fujiyama T, Kurihara K, Ito T, Honda T, Tokura Y. PD-1 expression defines epidermal CD8+ CD103+ T cells preferentially producing IL-17A and using skewed TCR repertoire in psoriasis. J Invest Dermatol 2021; 141: 2426-2435.e5.
6. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol 2008; 58: 826-850.
7. Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018.
8. López-Estebaranz JL, Sánchez-Carazo JL, Sulleiro S. Effect of a family history of psoriasis and age on comorbidities and quality of life in patients with moderate to severe psoriasis: results from the ARIZONA study. J Dermatology 2016; 43: 395-401.
9. Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician 2010; 82: 249-255.
10. Tramontana M, Bianchi L, Hansel K, Agostinelli D, Stingeni L. Nickel allergy: epidemiology, pathomechanism, clinical patterns, treatment and prevention programs. Endocr Metab Immune Disord Drug Targets 2020; 20: 992-1002.
11. Hughes JDM, Pratt MD. Allergic contact dermatitis and autoeczematization to Proctosedyl® cream and Proctomyxin® cream. Case Rep Dermatol 2019; 10: 238-246.
12. Chang MW, Nakrani R. Six children with allergic contact dermatitis to methylisothiazolinone in wet wipes (baby wipes). Pediatrics 2014; 133: e434-e438.
13. Singh S, Verma P, Chandra U, Tiwary NK. Risk factors for chronic and chronic-relapsing tinea corporis, tinea cruris and tinea faciei: results of a case-control study. Indian J Dermatol Venereol Leprol 2019; 85: 197-200.
14. Pippin MM, Madden ML, Das M. Tinea cruris [internet]. Treasure Island, FL: StatPearls Publishing, 2020.
15. Siesling S, Elferink MAG, van Dijck JAAM, Pierie JPEN, Blokx WAM. Epidemiology and treatment of extramammary Paget disease in the Netherlands. Eur J Surg Oncol 2007; 33: 951-955.
16. Simonds RM, Segal RJ, Sharma A. Extramammary Paget’s disease: a review of the literature. Int J Dermatol 2019; 58: 871-879.
17. Zhao Y, Gong X, Li N, Zhu Q, Yu D, Jin X. Primary extramammary Paget’s disease: a clinicopathological study of 28 cases. Int J Clin Exp Pathol 2019; 12: 3426-3432.
18. Belousova IE, Kazakov DV, Michal M, Suster S. Vulvar toker cells: the long-awaited missing link:
a proposal for an origin-based histogenetic classification of extramammary paget disease. Am J Dermatopathol 2006; 28: 84-86.
19. Ishizuki S, Nakamura Y. Extramammary Paget’s disease: diagnosis, pathogenesis, and treatment with focus on recent developments. Curr Oncol 2021; 28: 2969-2986.
20. Kang Z, Zhang Q, Zhang Q, et al. Clinical and pathological characteristics of extramammary Paget’s disease: report of 246 Chinese male patients. Int J Clin Exp Pathol 2015; 8: 13233-13240.
21. Preti M, Micheletti L, Massobrio M, Ansai S-I, Wilkinson EJ. Vulvar Paget disease: one century after first reported. J Low Genit Tract Dis 2003; 7: 122-135.
22. Morris CR, Hurst EA. Extramammary Paget disease: a review of the literature – Part I: history, epidemiology, pathogenesis, presentation, histopathology, and diagnostic work-up. Dermatol Surg 2020; 46: 151-158.
23. Kibbi N, Owen JL, Worley B, et al. Evidence-based clinical practice guidelines for extramammary Paget disease. JAMA Oncol 2022; 8: 618-628.
24. Ohara K, Fujisawa Y, Yoshino K, et al. A proposal for a TNM staging system for extramammary Paget disease: retrospective analysis of 301 patients with invasive primary tumors. J Dermatol Sci 2016; 83: 234-239.
25. Hata M, Koike I, Wada H, et al. Radiation therapy for extramammary Paget's disease: treatment outcomes and prognostic factors. Ann Oncol 2014; 25: 291-297.
Skin lesions
Anal and rectal diseases

