What’s the diagnosis?
An elderly man with an intensely itchy skin eruption
Case presentation
An 89-year-old man presents with a one-month history of an erythematous, papular eruption on his trunk and limbs. He describes the itch as ‘unbearable’ and it has been waking him at night. He has been prescribed topical corticosteroids, which have relieved his pruritus only temporarily.
The patient has a history of hypertension, atrial fibrillation and hypothyroidism, for which he takes irbesartan, apixaban, metoprolol and thyroxine. He has been otherwise well and does not have any other symptoms.
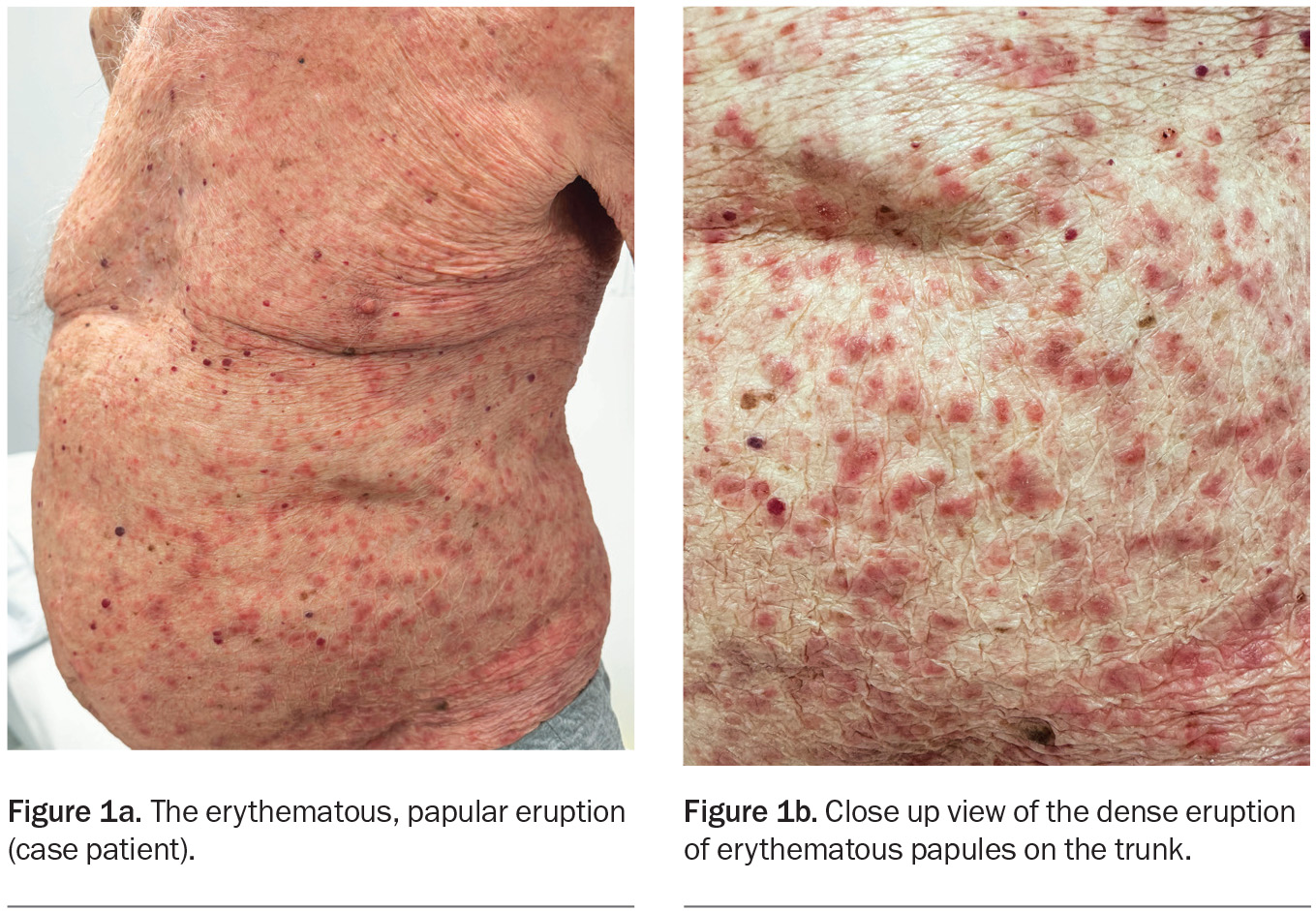
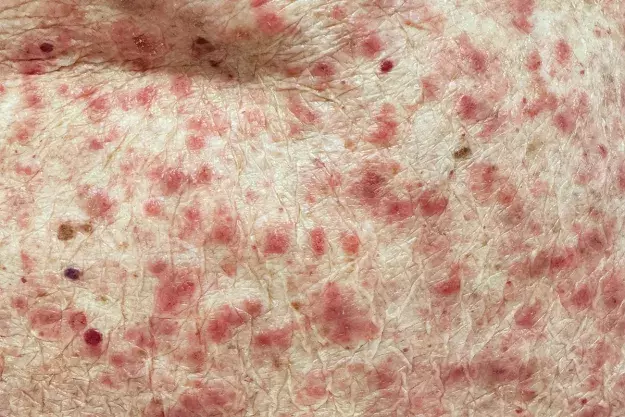
On examination, erythematous papules with a small amount of scale are observed on the patient’s neck, trunk and limbs (Figures 1a and b). His mucous membranes appear normal. No lesions are seen on the scrotum but he says that it is itchy. A punch biopsy has been performed, which showed ‘eosinophil rich dermatitis, macroscopic features non-specific’.
Differential diagnoses
Conditions to consider among the differential diagnosis include the following.
Grover’s disease
A relatively rare skin condition, Grover’s disease (transient and/or persistent acantholytic dermatosis) was first described in 1970.1 The characteristic lesions are erythematous papules, papulovesicles and small nodules on the trunk and proximal limbs. The eruption is often pruritic, and may be intensely pruritic. Men who are middle-aged or older are most commonly affected. The clinical course varies greatly and it may last a few weeks to several years.2
The exact pathogenesis of Grover’s disease is unknown but risk factors include xerosis, actinic skin damage, underlying skin disease (such as atopic dermatitis), immunodeficiency and prolonged bed rest. Common triggers include heat, sweating and exercise.3
Grover’s disease is usually a clinical diagnosis but may be confirmed by skin biopsy. Histologically, it is characterised by acantholysis (breakdown of intercellular connections between epidermal cells).4
This was not the correct diagnosis for the case patient. The distribution of his skin eruption was more widespread than is seen in Grover’s disease, and the histopathology results (eosinophil rich, no acantholysis) were not typical for this condition.
Bullous pemphigoid
Bullous pemphigoid, the most common autoimmune subepidermal blistering disease, usually affects older adults. It is generally caused by autoantibodies against proteins at the dermal-epidermal junction, which results in damage of the junction and leads to tense fluid-filled bullae.5 In rare cases, and especially in younger patients, bullous pemphigoid may be induced by medications – these include (but are not limited to) diuretics, antibiotics, NSAIDs and diabetes medications, specifically dipeptidyl peptidase 4 inhibitors, alpha-glucosidase inhibitors and meglitinides as well as sulfonylureas and nonsulfonylureas.6,7
Bullous pemphigoid typically begins as a nonspecific urticarial, papular or eczematous eruption with associated pruritus. About 20% of patients present in this prodromal phase, when making a clinical diagnosis is most difficult.8 Within weeks to months, it evolves to the bullous phase, which is characterised by tense blisters and bullae that contain clear fluid and sometimes blood. These eventually rupture, leaving behind erosions and crusts. There is an atypical variant, in which patients may not have any bullae.9 The eruption may affect any area of the body but is usually distributed on the trunk, axillae and flexor aspects of the proximal limbs.
If the typical bullae are present then bullous pemphigoid is usually a clinical diagnosis, but when there is doubt the diagnosis can be confirmed by biopsy. At least two samples should be taken: one for hematoxylin and eosin staining and another at the perilesional skin for direct immunofluorescence, which is the gold standard test for immunobullous disease. Histopathological characteristics include subepidermal splitting; direct immunofluorescence highlights IgG and C3 autoantibodies against the basement membrane zone.10
Although the case patient was in the usual age group for bullous pemphigoid, this was not the correct diagnosis. His skin eruption had remained static over one month and he had not developed bullae. Also, results of the histopathology did not show subepidermal splitting and showed more eosinophils than would be expected in this condition.
Prurigo nodularis
Prurigo nodularis (nodular prurigo) is a chronic skin disorder characterised by persistent, intense pruritus. It usually presents as well-circumscribed, firm papules and nodules. The severity can vary – patients may have a few lesions, or hundreds, of varying sizes. There is also variation in colour: lesions are typically flesh- or pink-coloured in fairer skin types and brown or black in darker skin types. Excoriations may be present due to chronic scratching.
Prurigo nodularis can occur in any location but is most common on the trunk and limbs in a symmetrical distribution.11 Both genders and all age groups can be affected.
The aetiology of prurigo nodularis is poorly understood but it is thought to be associated with the itch-scratch cycle. It has been shown to be associated with underlying chronic dermatoses (such as atopic dermatitis), internal diseases and various drugs (such as opioids, antimalarial medications and cancer treatments).12,13
Prurigo nodularis is usually a clinical diagnosis. If biopsy is performed, skin histopathology may show irregular epidermal hyperplasia and hyperkeratosis.14 Investigations to rule out other causes and underlying systemic diseases by a ‘pruritus screen’ are recommended for patients with chronic itch. This may include a combination of blood tests: full blood count; liver function test; electrolytes, urea and creatinine; calcium, magnesium and phosphate; thyroid stimulating hormone and parathyroid hormone levels; HIV test; hepatitis B and C; iron studies; erythrocyte sedimentation rate; protein electrophoresis or paraprotein typing; and assays for light chains. Investigations may also involve urine and stool tests (e.g. microscopy and culture, including tests for ova, cysts and parasites) as well as directed radiographic tests and, where appropriate, a workup for malignancy.15
Prurigo nodularis is not the correct diagnosis for the case patient, whose papular lesions appeared erythematous and were not firm to touch. In addition, his skin eruption was more widespread than is typical for this condition.
Scabies
This is the correct diagnosis. Scabies is a contagious disease caused by parasitic infestation of the Sarcoptes scabiei var. hominis mite within the skin. The mite burrows in the stratum corneum layer of the epidermis, where it lays eggs that hatch into larvae and mature into adults in around three weeks.16 Scabies is usually transmitted through skin-to-skin contact. Transmission via fomites, such as clothing or bedding, is rare.
In 2017, the WHO declared scabies a neglected tropical disease.17 Worldwide, at least 200 million people suffer from the disease at any one time, mostly in developing countries, and particularly in low-resource and low-socioeconomic areas with a high population density. Scabies is endemic in some Aboriginal and Torres Strait Islander communities in Australia, with rates as high as 33% in children.18 Unfortunately, this is associated with very high rates of cutaneous infections such as cellulitis and impetigo, caused by Staphylococcus aureus and Streptococcus species, which can be complicated by poststreptococcal glomerulonephritis and rheumatic heart disease. In metropolitan areas, family outbreaks may be initiated by children through close contact, such as in schools or at sleepovers, and large outbreaks may occur in residential aged care facilities.19
Scabies presents as an intensely pruritic, widespread, symmetrical skin eruption. These symptoms usually begin four to six weeks after infestation. The skin lesions vary greatly, from nodules and papules to generalised dermatitis.16
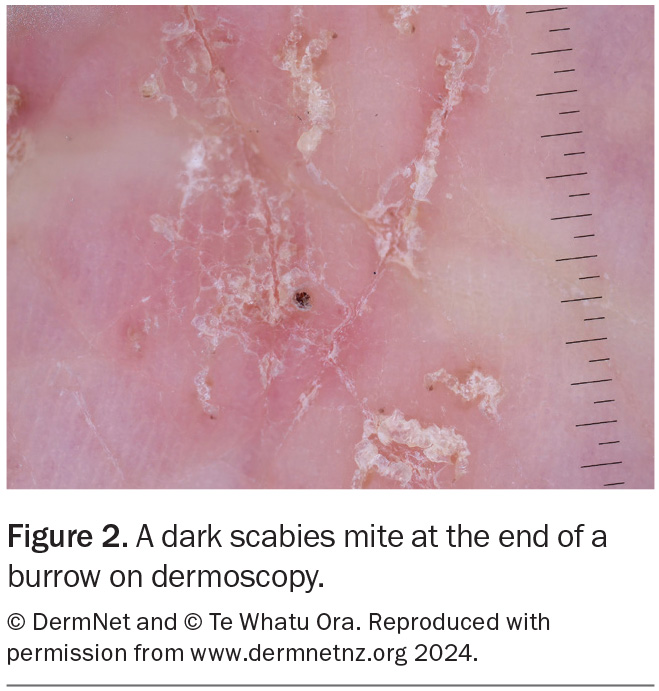
A diagnosis of scabies is usually made on the basis of clinical findings. Pathognomonic signs include mite burrows: linear or serpiginous thread-like tracks, commonly located on the hands (especially between the digits), wrists, intertriginous areas, axillae, buttocks and groin and genital areas.
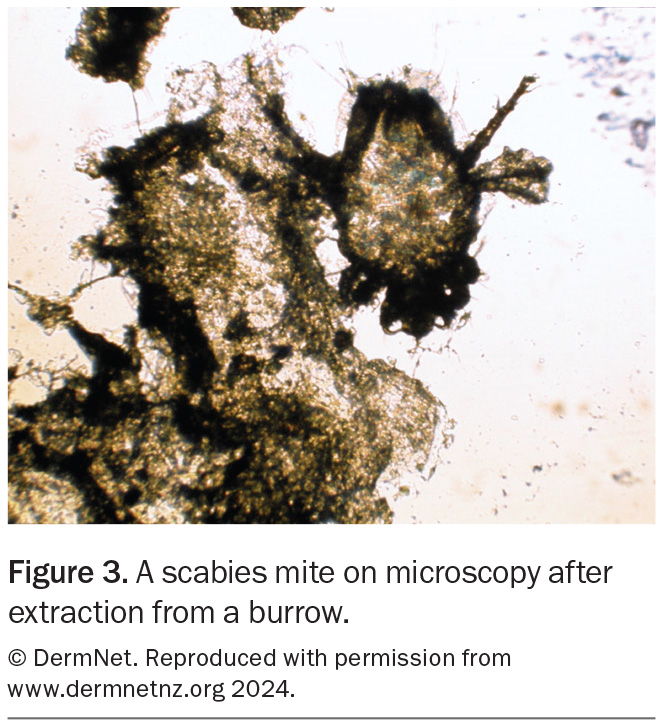
Dermoscopy can be useful for visualising burrows (Figure 2). Adult mites and their eggs or faeces can sometimes be directly visualised on microscopy of skin scrapings (Figure 3). Although rarely needed for diagnosis, a skin biopsy may reveal burrows or adult mites or their eggs, larvae or faeces. Pruritic nodules on the penis and scrotum in males and around the areolae in females are highly suggestive of the diagnosis.
Management
The successful eradication of scabies requires the use of general environmental measures combined with medical treatment. The patient’s clothing, towels and bedding – as well as those of household and close contacts – should be washed on a hot cycle (at least 60oC). Alternatively, these items can be placed in a sealed plastic bag for eight days, which allows time for any mites and their hatched eggs to die.19
Topical and oral medical treatment options are available for scabies eradication. The topical treatment of choice is permethrin 5% cream, which is applied from the neck down, covering the entire skin surface, including under the nails (using a nailbrush), but avoiding the eyes and mucous membranes.20 This treatment needs to be left on the skin for a minimum of eight hours, usually overnight. Permethrin should be avoided in infants younger than 2 months of age due to the risk of percutaneous absorption – instead, guidelines recommend use of a 5 to 7% sulfur preparation in soft white paraffin, applied topically once daily for three days.21,22 Second-line topical options include benzyl benzoate 25%, crotamiton 10% cream and sulfur 5 to 10% ointment.23-25 In situations where adherence may be an issue, oral treatment with ivermectin 200 mcg/kg (generally 12 mg in adults) taken with food may be preferred. For both oral and topical treatment, a second dose must be given in seven days to treat any mites that might have hatched from eggs.20
It is important to explain to the patient that pruritus may last for several weeks after successful treatment. Topical permethrin is very effective (with cure rates >98% after two applications).26 Oral ivermectin has been shown to be less effective than permethrin, with one randomised controlled trial showing that two doses of ivermectin is as effective as a single application of permethrin.27 However, ivermectin is sometimes preferred because it is more practical in some settings. Common reasons for treatment failure include not applying the topical treatment correctly, not repeating the treatment in seven days, not treating close contacts simultaneously and not following general measures for scabies eradication.28
Importantly, all close contacts of the patient should also have eradication therapy, regardless of symptoms, because it takes four to six weeks after infestation for symptoms to develop.20 For outbreaks in residential aged care facilities, all residents and close contacts of the patient require treatment, and quarantine measures should be placed.
Outcome
The case patient was diagnosed with scabies. The clinical presentation of an intensely pruritic, widespread, erythematous papular eruption was consistent with features of scabies and dermoscopic examination of lesions revealed typical burrows of the scabies mite. The patient lived with his wife and was visited by their daughter daily, and all three required eradication therapy (two doses of oral ivermectin, seven days apart). The need for general environmental measures was explained carefully and written instructions were provided.
At a two-week follow-up visit, the patient and his family had completed medical therapy (two doses of oral ivermectin each) and strictly adhered with the instructions given. His skin eruption and associated pruritus had improved considerably. He was advised that it is not uncommon for the itch to last several weeks. MT
COMPETING INTERESTS: None.
ACKNOWLEDGEMENT: Figures 2 and 3 reproduced with permission from www.dermnetnz.org 2024.
References
1. Grover RW. Transient acantholytic dermatosis. Arch Dermatol 1970; 101: 426-434.
2. Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis). Arch Pathol Lab Med 2009; 133: 1490-1494.
3. Quirk CJ, Heenan PJ. Grover’s disease: 34 years on. Australas J Dermatol 2004; 45: 83-88.
4. Davis MDP, Dinneen AM, Landa N, Gibson LE. Grover’s disease: clinicopathologic review of 72 cases. Mayo Clin Proc 1999; 74: 229-234.
5. Stanley JR, Tanaka T, Mueller S, Klaus-Kovtun V, Roop D. Isolation of complementary DNA for bullous pemphigoid antigen by use of patients’ autoantibodies. J Clin Invest 1988; 82: 1864-1870.
6. Stavropoulos PG, Soura E, Antoniou C. Drug‐induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol 2014; 28: 1133-1140.
7. Huang L, Liu Y, Li H, et al. Bullous pemphigoid and diabetes medications: a disproportionality analysis based on the FDA Adverse Event Reporting System.
Int J Med Sci 2021; 18: 1946-1952.
8. Di Zenzo G, Della Torre R, Zambruno G, Borradori L. Bullous pemphigoid: from the clinic to the bench. Clin Dermatol 2012; 30: 3-16.
9. Alonso-Llamazares J, Rogers RS 3rd, Oursler JR, Calobrisi SD. Bullous pemphigoid presenting as generalized pruritus: observations in six patients. Int J Dermatol 1998; 37: 508-514.
10. Weigand DA, Clements MK. Direct immunofluorescence in bullous pemphigoid: effects of extent and location of lesions. J Am Acad Dermatol 1989; 20: 437-440.
11. Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol 2020; 83: 1559-1565.
12. Williams KA, Huang AH, Belzberg M, Kwatra SG. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol 2020; 83: 1567-1575.
13. Lee MR, Shumack S. Prurigo nodularis: a review. Australas J Dermatol 2005; 46: 211-220.
14. Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol 2010; 37: 578-586.
15. Nowak D, Yeung J. Diagnosis and treatment of pruritus. Can Fam Physician 2017; 63: 918-924.
16. Hicks MI, Elston DM. Scabies. Dermatol Ther 2009; 22: 279-292.
17. World Health Organization. Scabies. May 2023. Available online at: https://www.who.int/news-room/fact-sheets/detail/scabies (accessed May 2024).
18. Gramp P, Gramp D. Scabies in remote Aboriginal and Torres Strait Islander populations in Australia: a narrative review. PLoS Negl Trop Dis 2021; 15: e0009751.
19. Hardy M, Engelman D, Steer A. Scabies: a clinical update. Aust Fam Physician 2017; 46: 264-268.
20. Therapeutic Guidelines. Considerations in the use of topical corticosteroids. 2022. Available online at: https://www.tg.org.au/ (accessed May 2024).
21. Albakri L, Goldman RD. Permethrin for scabies in children. Can Fam Physician 2010; 56: 1005-1006.
22. Therapeutic Guidelines. Presentation and diagnosis of scabies. 2022. Available online at: https://www.tg.org.au/ (accessed May 2024).
23. Meyersburg D, Hoellwerth M, Brandlmaier M, et al. Comparison of topical 5% permethrin vs. 25% benzyl benzoate in treating scabies – a double-blinded, randomized controlled study. Br J Dermatol 2023: 486-491.
24. Pourhasan A, Goldust M, Rezaee E. Treatment of scabies, permethrin 5% cream vs. crotamiton 10% cream. Ann Parasitol 2013; 59: 143-147.
25. Sharquie KE, Al-Rawi JR, Noaimi AA, Al-Hassany HM. Treatment of scabies using 8% and 10% topical sulfur ointment in different regimens of application.
J Drugs Dermatol 2012; 11: 357-364.
26. Chhaiya SB, Patel VJ, Dave JN, Mehta DS, Shah HA. Comparative efficacy
and safety of topical permethrin, topical ivermectin, and oral ivermectin in
patients of uncomplicated scabies. Indian J Dermatol Venereol Leprol 2012;
78: 605-610.
27. Usha V, Gopalakrishnan Nair TV. A comparative study of oral ivermectin and topical permethrin cream in the treatment of scabies. J Am Acad Dermatol 2000; 42(2 Pt 1): 236-240.
28. Sunderkötter C, Feldmeier H, Fölster‐Holst R, et al. S1 guidelines on the diagnosis and treatment of scabies – short version. J Dtsch Dermatol Ges 2016; 14: 1155-1167.
Parasitic diseases